
Historically, octopuses have been considered solitary, asocial individuals (Barbato et al., 2007; Hanlon and Messenger, 2018); however, recent studies suggest that octopuses use a unique and systematic arrangement of visual signals to communicate with conspecifics (Huffard, 2007; Caldwell et al., 2015; Scheel et al., 2016). These visual signals include chromatic and textural changes, postures, different forms of locomotion, and inking, which can be combined or used consecutively to create specific displays.
Some of the most complex signals octopuses use are chromatic signals. Since octopuses have direct neural control of pigment-containing cells, called chromatophores, octopuses can quickly change chromatic signals, adjust signal strength, and even perform bilateral signaling (Barbato et al., 2007; Hanlon and Messenger, 2018). Although colorblind, octopuses have excellent vision – consequently, by using highly contrasting chromatic signals, octopuses can clearly display their intent (e.g., to show dominance or submissiveness) toward a conspecific (Tricarico et al., 2011; Hanlon and Messenger, 2018).
Many visual signals that octopuses utilize are species-specific, therefore characterizing and documenting visual signals of octopuses via ethograms provides useful supplementary information for validating species identification (Barbato et al., 2007; Huffard, 2007). Ethograms are useful libraries of information that document the behaviors of organisms. For octopuses, ethograms can act as resources for scientists studying how ecological influences, such as conspecific interactions or habitat availability, may affect the evolution of signal development or communication.

Octopus rubescens is a subtidal species found along the west coast of North America (from Alaska to California), sheltering in kelp beds and rocky areas, and are commonly found in Admiralty Bay, WA (Cowles, 2005). The benthic habitat of this bay is generally barren and flat, characterized by mud, sand, and small rocks, with few hiding places for this non-burrowing octopus species. However, the bay is littered with discarded glass bottles which O. rubescens opportunistically use as dens (Anderson et al., 1999). Octopus rubescens have capitalized on this new habitat source which may have inadvertently concentrated individuals of this species within the bay (Chase and Verde, 2011). Consequently, O. rubescens may interact with conspecifics more frequently within this “artificial” environment and these interactions may be characterized by visual signals used by octopuses to communicate with each other.
Given that octopuses use visual signals to interact, the purpose of this study was to determine the frequency of such signals used by O. rubescens individuals to communicate with conspecifics, and to document those visual signals in an ethogram. As such, this study addressed the following questions:
1) What are the visual signals that O. rubescens use during interactions with conspecifics?
2) Is the frequency of interactions influenced by the sex of octopuses?
3) Do the type or frequency of visual signals differ between initiators and reactors of an interaction?
Methods
Octopus rubescens individuals were collected via SCUBA from Admiralty Bay, WA and housed at the Rosario Beach Marine Laboratory (RBML), Anacortes, WA. Individual octopuses were housed in plastic containers with constant flowing ambient seawater via a manifold system (Figure 1). Rocks were placed on top of the containers as additional measures to prevent octopuses from escaping. The total sample size (N) for this experiment was 20 octopuses (10 males & 10 females).

Figure 1. Plastic containers with constant flowing ambient seawater (via a manifold system) which housed individual octopuses; system was designed and fabricated by Chase and Verde (2011)
To identify the visual signals of O. rubescens, GoPro cameras (Figure 2) recorded videos of octopuses interacting for 15 min in an observation tank (Figure 3).
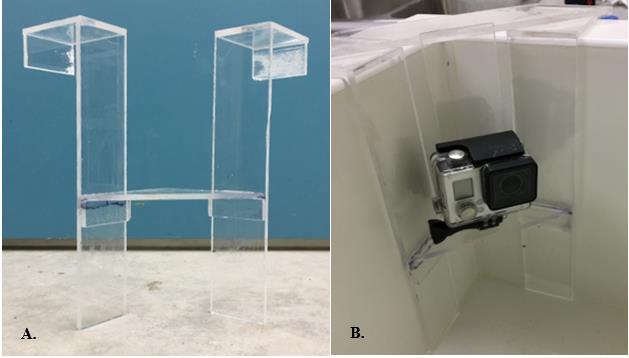
Figure 2. Fabricated plexiglass frames (A) that secured cameras (B) to the corners of observation tank.
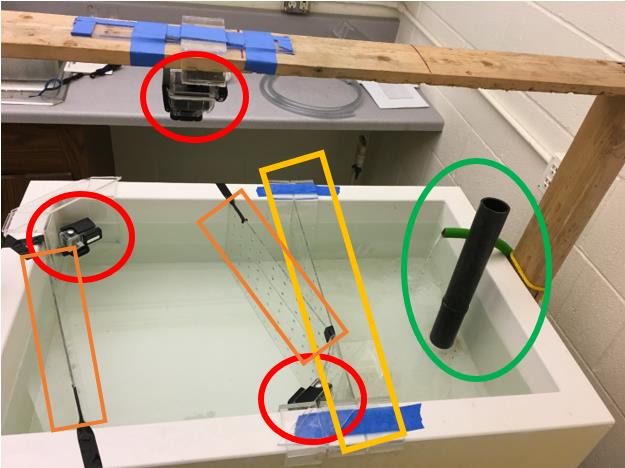
Figure 3. Experimental set-up for the study: a flow-through seawater observation tank was outfitted with three GoPro cameras (red circles). The tank was divided by a piece of plexiglass (larger, yellow rectangle) with holes drilled in it to buffer the rippling effect of the seawater in/outflow (green oval). Only half of the tank was used as the testing area for the octopuses. To eliminate blind spots for the corner cameras in the tank, two plexiglass dividers were cut and angled width-wise along the tank walls to narrow the space (smaller, orange rectangles).
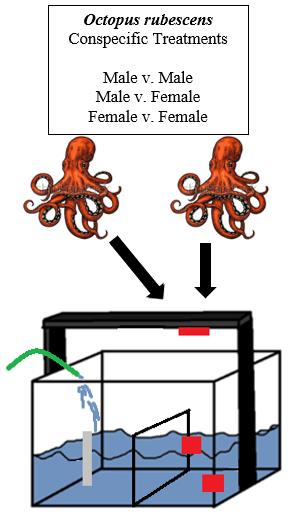
Each octopus was allowed to interact with all other octopuses of the same and opposite sex (Figure 4).
VLC Media Player was utilized to observe and analyze videos recorded by the GoPro cameras. Snapshots from the videos were used to create an ethogram. Any octopus interaction that lasted at least 5 s was analyzed for the use of visual signals (chromatic, textural, locomotor, and postural).
Results
Octopus rubescens used a variety of visual signals to communicate and interact with conspecifics during the 15 min trials. These visual signals included chromatic (Figures 5 & 6), textural (Figure 7), inking (Figure 8), locomotor (Figure 9), and postural cues (Figure 10). The frequency of interactions differed by 1 interaction per test between the M/M octopus combinations (5.2 interactions per test) and M/F and F/F octopus combinations (4.2 interactions per test).
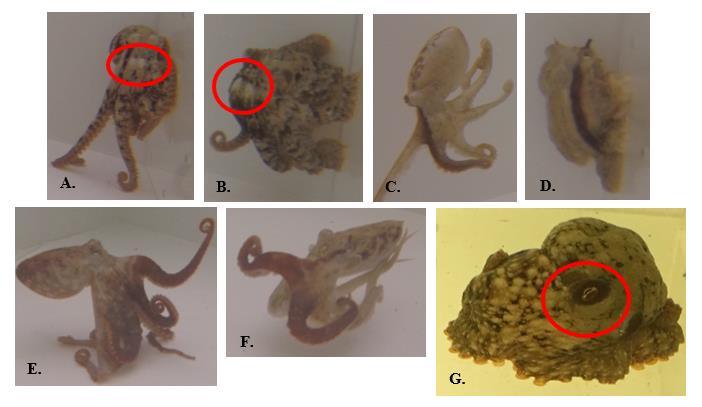
Figure 5. Partial-body chromatic signals used by O. rubescens. A.-B. False frontal white eye spots: two adjacent white spots centered below eyes; C.-D. Dark longitudinal stripe(s): typically run(s) from eye down first left and/or right arm(s); does not always run length of arm; E.-F. Darkened arms: typically first left or right (or both) arms of octopus; all arms can be darkened; G. Dark eye rings: darkened patch encircling eyes.

Figure 6. Full-body chromatic signals used by O. rubescens. A. Pale: body is light ochre to gray or white; B.-C. Mottled ochre: ochre/sandy-colored body; white and/or brown/black spots (spot density and color varies) across entire body; D. Dark ochre: completely darkened body, red to brown/dark ochre; E.-F. Deimatic: dark spots/patches on mantle, pale arms; G. Ochre: ochre/sand-colored body (some variation in darkness); H.-J. Intense mottle: high contrast between dark and pale markings on body, bars/bands of dark along arms may be present; often papillate.
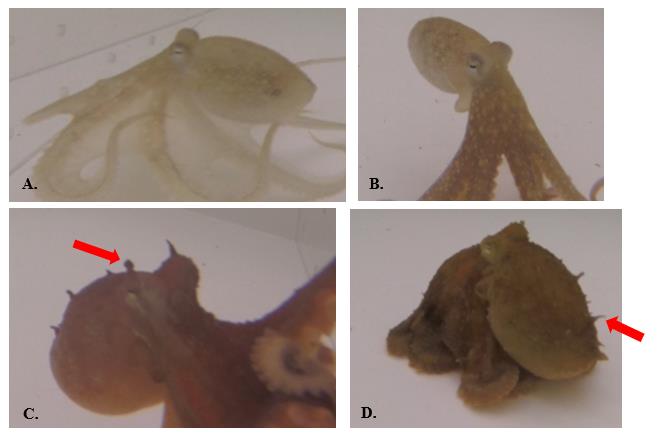
Figure 7. Textural signals used by O. rubescens. A.-B. Smooth: no papillae; C.-D. Papillate: papillae visibly raised.

Figure 8. Inking signal used by O. rubescens. Inking was recorded as either present or absent.
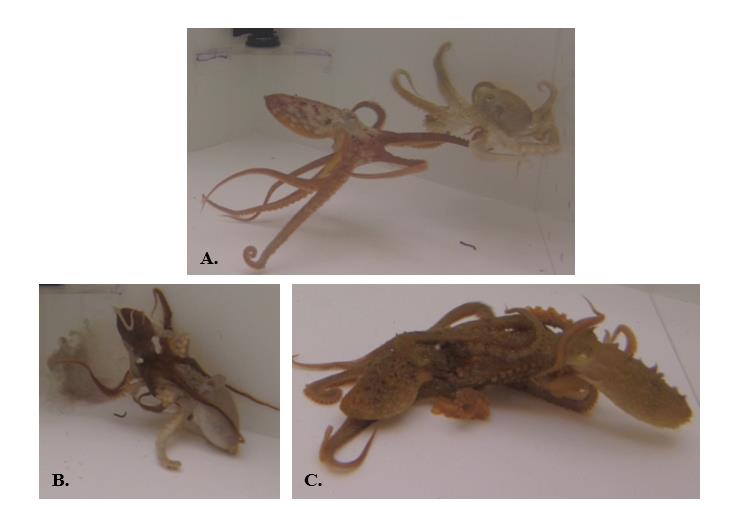
Figure 9. Locomotor signals used by O. rubescens. A. Attack: octopus launches self at conspecific; forward rush; jet propulsion commonly utilized; B.-C. Grappling: octopuses entangled in each other’s arms, reaching, biting, grabbing. Signals not visualized: Stationary, Threaten, Flee, Chase, Approach.

Figure 10. Postural signals used by O. rubescens. A. Spreading arms: arms stretched out; B. Flattened: low to bottom, mantle lowered; C. Beak-to-beak: octopuses facing each other, touching close to beaks; D. Reaching: one/multiple arms reaching for conspecific; E. Upright: alert toward conspecific; F. Jetting: arms together, typically, but can be curled; G: Loose arms: arms hanging loosely around/below body, can be slightly curled; H. Stand tall: upright, arms straightened to make self taller/larger; I. Grappling: octopuses fighting; J. Attack: arms poised to attack conspecific (two front arms typically curled and held up); often combined with chromatic signal ‘Darkened arms’; K. Raised arms: arms raised, often curled; typically front arms; L. Crawling: arms out, loose or curled, propelling octopus; M. Curled arms: arms curled tightly against body.
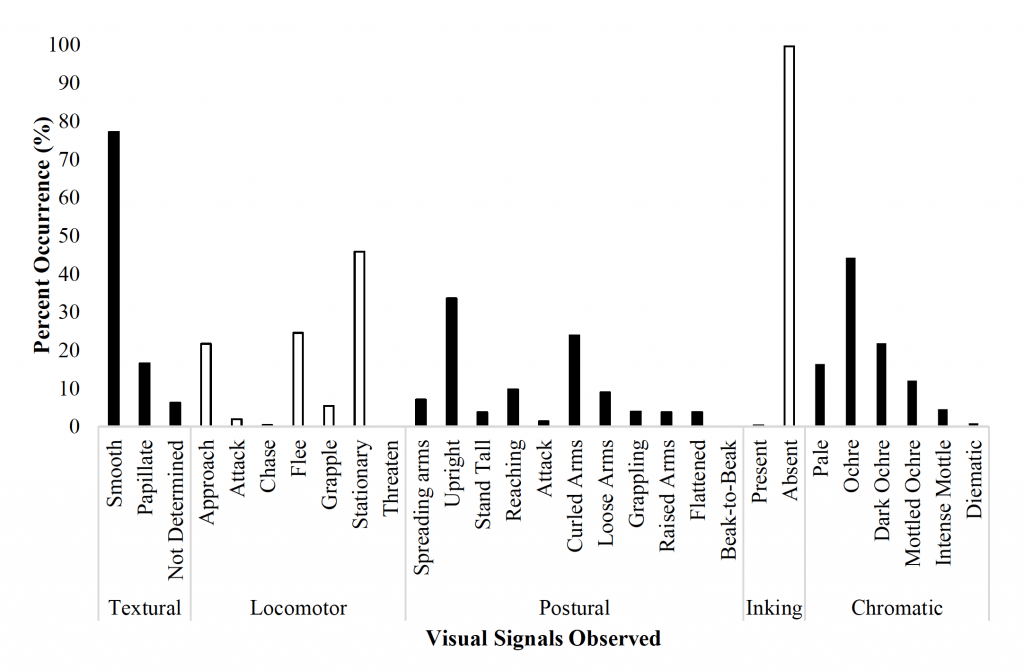
Figure 11. The percent occurrence of the most common visual signals displayed by O. rubescens during interactions with conspecifics in an observation tank (Nmale = Nfemale = 10). The five categories of signals include textural, locomotor, postural, inking, and chromatic which all have a variety of subcategories.
Octopus interactions (Figure 11) were typically characterized by the locomotor signals ‘stationary’ (45.9%), ‘approach’ (21.7%), and ‘flee’ (24.6%) by one or both octopuses for all sex combinations (M/M, M/F, F/F). The most common chromatic signals included ‘ochre’ (44.3%), ‘dark ochre’ (21.9%), and ‘pale’ (16.4%); octopuses appeared to primarily use ‘ochre’ as a resting pattern of pigmentation. The most common postures included ‘upright’ (33.6%) and ‘curled arms’ (23.9%). Textural signals were predominantly ‘smooth’ (77.2%) and octopuses rarely inked.
Interactions between octopuses were also divided between the initiator and reactor within each sex combination (M/M, M/F, F/F) and these results can be viewed in the full manuscript, provided in a link at the end of this post.
Discussion
The ethogram produced in this study portrays a variety of visual signals that O. rubescens used during conspecific interactions and suggests that communication was occurring between individuals. The number of interactions per test for all sex combinations (M/M, M/F, F/F) of octopus was similar which suggests that sex had little influence on the frequency of interactions between octopuses. While O. rubescens may gather in an area for a specific habitat resource, such as the bottles used as dens in Admiralty Bay, the species demonstrated predominantly aggressive behavior toward conspecifics during this study, suggesting that they are not a social species even if they are not solitary.

Nonetheless, visual signals are especially important for octopuses to use during aggressive interactions because they can clearly display an octopus’s intentions to attack or submit, depending on the likelihood of winning or losing a fight (Barbato et al., 2007; Scheel et al., 2016). Having the ability to display such intent helps octopuses avoid unnecessary harm.
One aggressive signal, ‘grappling’, was not as frequent as other postural or locomotor signals, but did occur during interactions and most frequently between males. Increased aggression between males perhaps could be attributed to their need to compete for females in their natural habitat.
 Although the behaviors documented were in a laboratory setting, the ethogram produced in this study may still serve as a useful reference. Future studies can document the visual signals of O. rubescens collected from other habitats and reveal potential variations in visual signals used by this species. Since the sample of octopuses used in this study was from a population in Admiralty Bay, these octopuses may use a specialized system of visual signaling during interactions, as opposed to more solitary octopuses. This may allow scientists to hypothesize that the visual signals used by O. rubescens are influenced by surrounding habitats, like Admiralty Bay, or population density. The basic ethogram created in this study can also act as an additional resource of comparison between octopus species, regardless of the fact that the visual cues identified were during conspecific interactions.
Although the behaviors documented were in a laboratory setting, the ethogram produced in this study may still serve as a useful reference. Future studies can document the visual signals of O. rubescens collected from other habitats and reveal potential variations in visual signals used by this species. Since the sample of octopuses used in this study was from a population in Admiralty Bay, these octopuses may use a specialized system of visual signaling during interactions, as opposed to more solitary octopuses. This may allow scientists to hypothesize that the visual signals used by O. rubescens are influenced by surrounding habitats, like Admiralty Bay, or population density. The basic ethogram created in this study can also act as an additional resource of comparison between octopus species, regardless of the fact that the visual cues identified were during conspecific interactions.
Link to full manuscript - Download PDF
Literature Cited
Anderson, R., P. Hughes, J. Mather, and C. Steele, C. 1999. Determination of the Diet of Octopus rubescens Berry, 1953 (Cephalopoda: Octopodidae), Through Examination of its Beer Bottle Dens in Puget Sound. Malacologia, 41:455–460.
Barbato, M., M. Bernard, L. Borrelli, and G. Fiorito. 2007. Body Patterns in Cephalopods: ‘‘Polyphenism’’ as a Way of Information Exchange. Pattern Recognition Letters, 28:1854–1864.
Caldwell, R., R. Ross, A. Rodaniche, and C. Huffard. 2015. Behavior and Body Patterns of the Larger Pacific Striped Octopus. PLoS One, 10: e0134152.
Chase, E, and A. Verde A. 2011. Population Density and Choice of Den and Food Made by Octopus rubescens Collected from Admiralty Bay, Washington, in July 2011. American Academy of Underwater Sciences, 30:110–116.
Cowles, D. 2005. Octopus rubescens Berry, 1953
Hanlon, R. and J. Messenger. 2018. Cephalopod Behaviour. Cambridge (England): Cambridge University Press.
Huffard, C. 2007. Ethogram of Abdopus aculeatus (D’Orbigny, 1834) (Cephalopoda: Octopodidae): Can Behavioral Characteristics Inform Octopodid Taxonomy and Systematics? Journal of Molluscan Studies, 73:185–193.
Scheel, D., P. Godfrey-Smith,and M. Lawrence. 2016. Signal Use by Octopus in Agonistic Interactions. Current Biology, 26:1–6.
Tricarico, E., L. Borrelli, F. Gherardi, and G. Fiorito. 2011. I Know my Neighbour: Individual Recognition in Octopus vulgaris. PLoS One, 6: e18710.
Authors: Rachel L. Borisko, Kirt L. Onthank, E. Alan Verde




